Abstract
Background: Langerhans Cell Histiocytosis (LCH) is a rare inflammatory neoplasm originating from bone marrow-derived CD207+/CD1+ clonal dendritic cells, with cells expressing BRAF V600E in around 50% of cases. Although more common in the paediatric setting, LCH is rare in adults, with an incidence of 1 to 2 cases per million. This combination of disease heterogeneity and infrequency means that data on treatment strategies and outcomes is limited, and a consensus on management is lacking. Data has historically been extrapolated from the larger paediatric cohort, with emerging phase II data on the efficacy and safety of upfront methotrexate and cytosine arabinoside (AraC) in adult LCH. Other agents used include cladribine (CdA), hydroxyurea, vinblastine, and prednisolone-based regimens, and more recently MEK/BRAF inhibitors, but none of these have been trialled prospectively. LCH can be an aggressive disease with a poor prognosis.
Methods : We included patients aged ≥18 years diagnosed with LCH on histopathology between January 1, 2000 and August 1, 2021 from ten sites across Australia and New Zealand. Variables included demographic data, system and organ involvement, central nervous system (CNS) involvement, bone marrow involvement and BRAF V600E mutation status. Outcomes measured included overall survival (OS), progression-free survival (PFS) and total lines of therapy. Response to therapy was modified from the Scoring System of the HS LCH III trial: Complete response (CR) was defined by resolution of all signs of disease activity, including neuroendocrine dysfunction, skin and radiological lesions; and a partial response (PR) was defined as patients with "active disease better" and those with "mixed response". Stable or worsening symptoms/radiology were classified as progressive disease (PD). Patients who were alive or lost to follow-up were censored on the date of last follow-up.
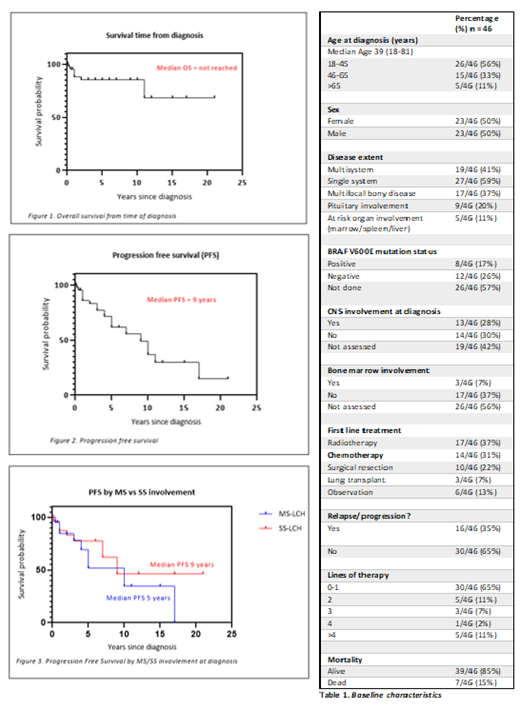
Results : We identified 46 patients with a median age at diagnosis of 39 years (range 18-81) (Table 1). The sex ratio was 1. 41% of patients had MS-LCH at diagnosis, of which 24% had high risk organ (liver, bone marrow, or spleen) involvement. 37% had multifocal bone disease. 28% of patients had CNS involvement at diagnosis. BRAF V600E mutation status was assessed in only 43% of cases, with a mutation frequency of 40%. The median number of lines of therapy was 1.7 (range 0-9). Three patients (7%) underwent lung transplantation for isolated pulmonary LCH. 31% percent of patients were treated with chemotherapy upfront, with the majority receiving AraC (43%,) and the remainder receiving vinblastine, prednisolone and 6-mercaptopurine (6-MP) (21%), vinblastine/prednisolone (21%), CdA (14%) or methotrexate and 6-MP (2%). Of note, 67% (n=4) of patients who were treated with upfront AraC and 100% (n=2) of patients treated with upfront CdA had a CR, while those patients treated with upfront vincristine-based regimes had, at best, a PR (50%, n=3) or PD (50%).
Of the 46 patients, with a median follow up of 34.6 months (range 0.63-250.2), there were 7 deaths (15%) with 6/7 due to progressive disease, and the remaining death due to complications of lung transplantation. Median OS was not reached (Figure 1), while OS at 5 years was 85%. Median PFS was 9 years (range 0.05-21) (Figure 2), with a non-significant trend towards reduced PFS in patients with MS-LCH (5 years) compared to SS-LCH (9 years) (Figure 3). There was also a trend towards reduced OS in patients who received AraC as systemic therapy versus other forms of systemic therapy.
Conclusion: We report the largest multicentre Australasian cohort of patients diagnosed with adult-onset LCH. OS and PFS were longer than previously reported in the literature. There remains significant heterogeneity in diagnostic and treatment strategies. There was a trend towards improved response rate to upfront AraC or CdA when compared to the vinblastine-based regimens, and further prospective research with these agents is required in this setting. Progress in this disease is challenging due its rarity, highlighting the need for standardisation of diagnostic and treatment strategies as well as a national and/or international registry.
Fong: Amgen: Research Funding; AbbVie, Amgen, Novartis, Pfizer, Astellas: Honoraria; Amgen, BMS: Speakers Bureau. Ku: Antegene: Consultancy; Roche: Consultancy; Genor Biopharma: Consultancy. Cunningham: Principia Biopharma: Research Funding; Rigel: Research Funding; Janssen: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Amgen: Research Funding; Astex: Research Funding; Celgene: Research Funding. Hamad: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal